12
2024
-
11
Enhance R&D service capabilities and focus on comprehensive enterprise needs
Jilin Chongming Biotechnology Co., Ltd. was established in November 2020 and focuses on the development, operation, and market promotion of in-vitro diagnostic products.
Author:
Company Introduction
Jilin Chongming Biotechnology Co., Ltd. was established in November 2020, focusing on the development, operation, and marketing of in-vitro diagnostic products. The company has a 1100-square-meter R&D center and a 1600-square-meter integrated factory. It meets the technical development of multiple platforms such as molecular diagnostic technology platform, immunochromatographic technology platform, and pathological analysis technology platform, which can be used for pilot production of products and provide sufficient basic conditions for transfer production.
Based on the established technology platform, the company has conducted in-depth research and product development in molecular diagnostics, pathology diagnostics, and immunochromatography. Currently, more than ten products have been successfully launched into the market and are widely used in various scenarios.
Currently, the company undertakes various forms of CDMO projects. Chongming Bio aims to create its own Brand while providing services to other R&D companies, committed to providing one-stop solutions from product incubation to registration.


Production Unit
The production unit includes: 100,000-level clean room, quality inspection laboratory, microbial limit test room, positive control room, etc.


R&D Center
The R&D center includes: PCR laboratory, physical and chemical laboratory, etc.
02Service Resources and Function Display
![]() Current Status of CDMO Development
Current Status of CDMO Development
Contract Development and Manufacturing Organization (CDMO) integrates R&D and production to provide enterprise customers with process R&D, optimization, formulation development, and pilot production services needed for innovative drug production.
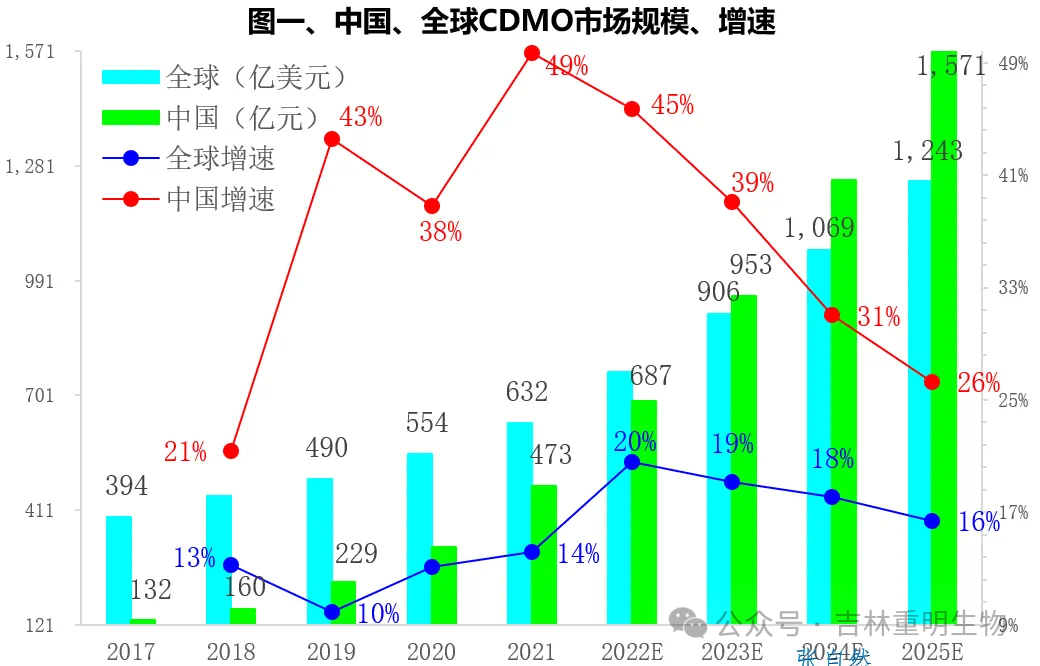
Currently, the global CDMO industry is still in a period of rapid development. According to Sullivan's statistics, the global CDMO market size has grown from US$39.4 billion in 2017 to US$63.2 billion in 2021, and will reach US$124.3 billion by 2025.
The wave of pharmaceutical innovation that began in 2015 has promoted the rapid development of China's CDMO industry. The size of China's CDMO market has grown from 13.2 billion yuan in 2017 to 47.3 billion yuan in 2021, a 2.6-fold increase in four years, with a compound annual growth rate of 37.7%, twice the compound growth rate of 18.5% for the global CDMO during the same period. It is expected to reach 157.1 billion yuan by 2025.
China's share of the global CDMO market has also increased from 5% in 2017 to 13% in 2021, and will account for one-fifth of the global market by 2025. The data in the figure above comes from the "Sullivan 2022 CDMO Industry Development Status and Future Trend Research Report".
![]() Core Advantages of CDMO Enterprises
Core Advantages of CDMO Enterprises
The core value of CDMO enterprises lies in the ability to deeply integrate their own high-tech added-value process R&D capabilities with large-scale production capabilities, and through clinical pilot production and commercial production supply models, deeply connect with customers' entire supply chain system from R&D, procurement to production, helping customers improve new product R&D efficiency and reduce new product R&D and production costs. In order to fully realize its core value, CDMO enterprises need to scientifically combine and flexibly apply a series of high-difficulty synthesis, purification and other specific technologies.
![]() Chongming Bio CDMO Services
Chongming Bio CDMO Services
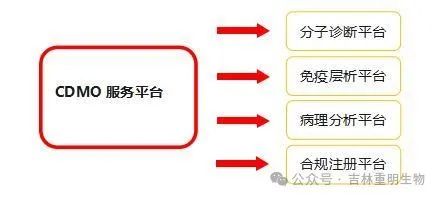
1. Molecular Diagnostics Platform
Molecular diagnostics refers to the use of molecular biology methods to detect changes in the structure or expression level of genetic material in a patient's body to make a diagnosis. Molecular diagnostics mainly refers to the detection of genes encoding various structural proteins, enzymes, antigens and antibodies, and immunoactive molecules related to diseases.
Currently, the molecular diagnostic technology team has established a complete R&D system and production process for some molecular diagnostic products, including multiple qPCR, methylation, ARMS, FISH, plasmid construction, pseudotype packaging reaction systems, some gene product regulation tools, etc.




Picture shows: Molecular diagnostic platform experimental instruments
2. Immunochromatographic Platform
Immunochromatographic technology, also known as lateral flow immunoassay, is a method that uses a known antigen or antibody pre-coupled to a carrier as a capture line. The sample to be tested is driven by the capillary action of the carrier to flow through the capture line. If the sample contains the corresponding antibody or antigen, it will specifically react with the antigen or antibody on the carrier and be adsorbed to the carrier, and the presence or content of the analyte can be determined by the color or luminescence of the marker. This method has the characteristics of specificity, simple operation, and speed, and is widely used in clinical diagnosis, environmental monitoring, food safety and other important fields.
Currently, the immunotechnology team has mastered the R&D and production processes of immunochromatographic technology using colloidal gold, colored microspheres, fluorescent microspheres, and magnetic microspheres as carriers.



Picture shows: Immunochromatographic platform experimental instruments
3. Pathology Analysis Platform
Biomarkers are biomolecules that can reflect the state or disease condition of a living organism, such as proteins, nucleic acids, and metabolites. The main labeling methods include enzyme labeling, fluorophore labeling, isotopic labeling, and biotin labeling. At present, biomarker detection technology has become an important means of clinical diagnosis, treatment, and prognosis assessment.
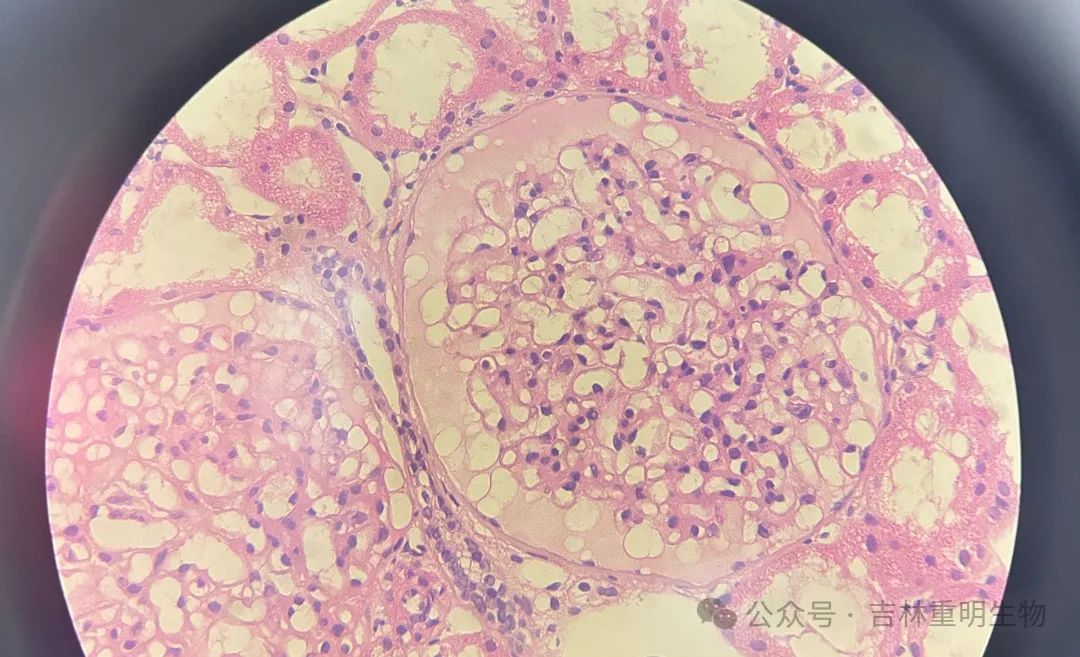
Picture shows: Immunohistochemical technology


Picture shows: Pathology analysis platform experimental instruments
4. Compliance Registration Platform
The platform has a group of professional registration personnel, covering six major aspects: design and development, pilot production, registration testing, clinical trials, registration application, and system audit. It integrates innovation, saving, professionalism, and efficiency to provide enterprises with professional customized services, including but not limited to registration material writing, system establishment, clinical plan design, clinical monitoring, on-site guidance, etc., building a fast track for enterprise development.
Platform Advantages
(1) Professional R&D team can help enterprises break through technical barriers and quickly solve R&D problems.
(2) Professional laboratories can reduce upfront investment and provide proof of concept for clinical and scientific researchers, enabling rapid scale-up pilot production of products.
(3) Professional registration team has rich experience in registration applications and system audits, avoiding repeated revisions.
In addition to the above services, our company can also provide contract manufacturing to achieve one-stop service! We have a mature system, sufficient talents, and standardized production workshops. Enterprises no longer need to build factories and equipment and other hardware facilities on their own, saving time, funds, and labor costs, and investing them in product improvement and design to improve product quality!
Our company's CDMO team hopes that, whether it's clinical researchers or various enterprises, only an idea is needed to drive the entire project R&D and production process through the company's efforts, greatly reducing the threshold for innovation transformation. At the same time, our company is committed to working with customers to create value and promote the continuous progress of in vitro diagnostic reagent enterprises.
Key words:
Related news
Tel / WhatsApp
+86-18088661155
Technical Support
Address
5th Floor, F8,Workshop 8, Science & Technology Incubation Park, Xinglong Comprehensive Bonded Zone, Changchun City, Jilin Province, China.

Service Hotline/Whatsapp:
E-mail:

